1. Describe the hazards of ionising radiation to living things.
All types of radiation (alpha, beta, and gamma) can damage living cells because they can ionise. Ionising radiation breaks down molecules into ions. Chemical reactions in living cells can be affected by these ions. This can cause living cells to die, or mutate and become cancerous.
2. Describe how radioactive materials are handled, used, and stored in a safe way to minimise the effects of these hazards.
●You should stay away from radioactive materials, avoiding the eyes. If possible, you should be protected from them, and use appropriate equipment to handle them. For example, tongs.
● Radioactive materials are stored in containers lined with lead to make sure that it is not exposed to the environment outside.
● Film badges can detect radiation; they will turn black to measure the amount of radiation a person is exposed to. The badges should be checked regularly to make sure the person is not experiencing too much radioactivity.
● Wear goggles while dealing with radioactive materials, and wash hands after using them.
● Limit the amount of time exposed to radiation.
● Look out for the radioactive hazard symbol.
Unit 15.6: The Nuclear Atom
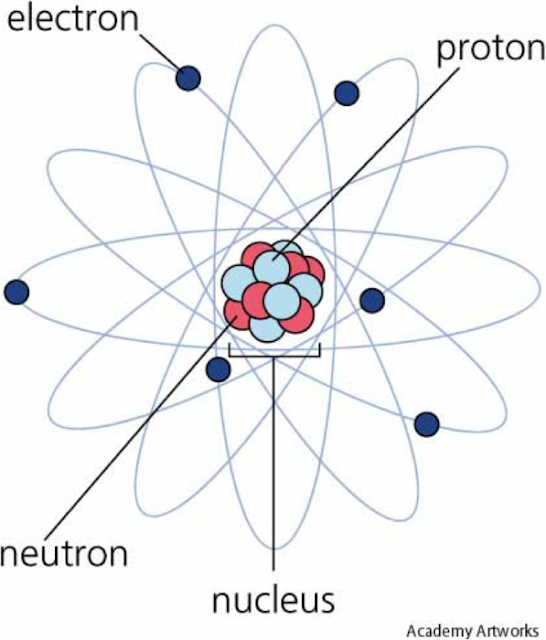
1. Describe the composition of the nucleus in terms of protons and neutrons.
In an atoms nucleus, you have protons and neutrons. Electrons are located on the outside of the nucleus.
2. Use the term proton number Z.
The proton number is the number of protons you have in an atom. For example, let's take a look at oxygen.
3. Use the term nucleon number A.
The nucleon number is the number of protons and neutrons combined. Using the diagram from above, we see that since oxygen has the proton number of 8 and the nucleon number of 16, it means that in oxygen there are 8 neutrons. The neutron number is also called the atomic mass.
Unit 15.7: Isotopes
1. Use the term isotope.
Some elements have more neutrons than others and they have various versions. These versions are called isotopes. The chemical properties are the same, but they have different masses due to the different amounts of neutrons. The number of protons, however, are the same, as a different number of protons would make it a different element.
2. Give and explain some practical uses of isotopes.
Take chlorine for an example. Its atomic mass is 37.5, because they have put the mean of the isotopes onto the Periodic Table. Isotopes are uses in medical therapy. For example, Cobalt 60 (as in 60 neutrons) is used to treat cancer and sterilise equipment.
3. Use the term nuclide and use the nuclide notation.
A nuclide is an atom or nucleus characterised by its number of protons and neutrons.
Unit 15.6: The Nuclear Atom
1. Describe the composition of the nucleus in terms of protons and neutrons.
In an atoms nucleus, you have protons and neutrons. Electrons are located on the outside of the nucleus.
2. Use the term proton number Z.
The proton number is the number of protons you have in an atom. For example, let's take a look at oxygen.
As seen from the diagram above, oxygen has 8 protons; the proton number can also be called the atomic number.
3. Use the term nucleon number A.
The nucleon number is the number of protons and neutrons combined. Using the diagram from above, we see that since oxygen has the proton number of 8 and the nucleon number of 16, it means that in oxygen there are 8 neutrons. The neutron number is also called the atomic mass.
Unit 15.7: Isotopes
1. Use the term isotope.
Some elements have more neutrons than others and they have various versions. These versions are called isotopes. The chemical properties are the same, but they have different masses due to the different amounts of neutrons. The number of protons, however, are the same, as a different number of protons would make it a different element.
2. Give and explain some practical uses of isotopes.
Take chlorine for an example. Its atomic mass is 37.5, because they have put the mean of the isotopes onto the Periodic Table. Isotopes are uses in medical therapy. For example, Cobalt 60 (as in 60 neutrons) is used to treat cancer and sterilise equipment.
3. Use the term nuclide and use the nuclide notation.
A nuclide is an atom or nucleus characterised by its number of protons and neutrons.
Above is a diagram explaining nuclide notation. Nuclide notation is what you would see on the Periodic Table.




If you declare with your mouth, “Jesus is Lord,” and believe in your heart that God raised him from the dead, you will be saved. Romans 10:9
ReplyDeleteThank you very much for your blog! It has really helped me, Lizzy Milne
GOD BLESS YOU YOU ARE MY LORD AND SAVIOUR NOW
ReplyDelete