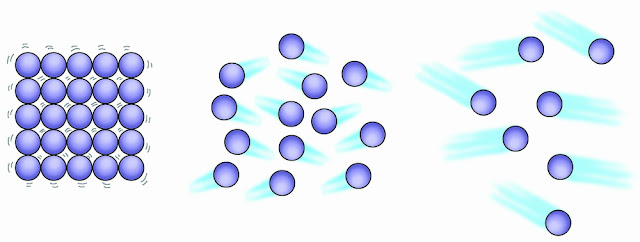
Unit 4.1 States of Matter
1. State the distinguishing properties of solids, liquids and gases.
| Solid | Liquid | Gas |
|---|---|---|
| Particles are tightly packed in a regular pattern | Particles are loosely bonded with gaps between them | Particles are completely seperated |
| Particles vibrate together, keeping its shape and position | Particles vibrate and slide past one another | Particles are free to move |
| Retains a fixed volume and shape | Takes shape of its container | Takes shape of its container |
| Does not flow easily | Flows easily | Flows easily |
source
Unit 4.2 Molecular Model
1. Describe qualitatively the molecular structure of solids, liquids and gases.
As mentioned above, particles in solids are in fixed positions, while particles in liquids and gases are more free.
2. Relate the properties of solids, liquids and
gases to the forces and distances between
molecules and to the motion of the
molecules.
In solids, the particles are close together because they are bonded in fixed positions. In liquids, the particles are attached together but are not as rigorously held together as particles in a solid. In gases, the molecules are completely unattached so the space between particles can be far and wide.
3. Interpret the temperature of a gas in terms
of the motion of its molecules.
When a gas is hot, its particles move quickly and collide often. This is because heat energy is given to the particles, giving them the kinetic energy to move. The hotter the gas, the faster the particles will move.
4. Describe qualitatively the pressure of a gas
in terms of the motion of its molecules.
Imagine you've got a sealed container containing gas. When the gas particles move, they hit the sides of the container; this creates pressure on the container. The pressure of the gas depends on how often and how hard the molecules are colliding with the inside of the container.
5. Describe qualitatively the effect of a
change of temperature on the pressure of
a gas at constant volume.
You know that a high temperature = faster motion and faster motion = more pressure. Therefore, the higher the temperature of gas, the more pressure of the gas at constant volume.
Unit 4.3 Evaporation
1. Describe evaporation in terms of the
escape of more energetic molecules from
the surface of a liquid.
Evaporation occurs when there are particles in a liquid that move faster, so fast that if they are near the surface they have enough energy to escape and become a gas.
2. Demonstrate understanding of how
temperature, surface area and air flow over
a surface influence evaporation.
Temperature: a higher temperatures means that the particles have more energy to escape, resulting in a faster rate of evaporation.
Surface area: with a bigger surface area, more of the molecules are at the surface, allowing them to escape.
Air flow: air flow picks up molecules at the surface before they can become liquid again. The higher rate of air flow, the faster the evaporation.
3. Relate evaporation to the consequent
cooling.
When water evaporates, it takes some thermal energy from whatever is has been on, resulting in that thing being cooler. Faster particles escape first, so slower particles are left behind; this means the temperature is lower than before.
Unit 4.4 Pressure Changes
1. Relate the change in volume of a gas to
change in pressure applied to the gas at
constant temperature and use the equation
pV = constant at constant temperature.
Boyle's law states that pressure and volume are inversely proportionate when the gas is at a constant temperature. Lowering the volume will increase in a higher pressure, and vice versa. This is because if a gas has a smaller volume, there is less space for the particles to move; they hit the sides of the container more frequently, resulting in higher pressure.




Excellent work. Helped a lot a lot a lot for studies
ReplyDeleteThis is soon helpful!!! Make more!!!!
ReplyDeletetypo soooooo
ReplyDeleteLove thissssssss, Please make more of these cause it helped so much.
ReplyDeleteDid you make one for Thermal Properties and Temperature?
ReplyDeletewhoever made this is a bloody legend
ReplyDeleteThank you very much. I am using it for my Unit 3 Physics students and will reference this page in the lecture notes.
ReplyDeletei cannot thank you enough
ReplyDeleteThx u saved me 💯
ReplyDeleteExtremely helpful! Thank you :)
ReplyDeletezxx
ReplyDelete